Protect your family from respiratory illnesses. Schedule your immunization here >
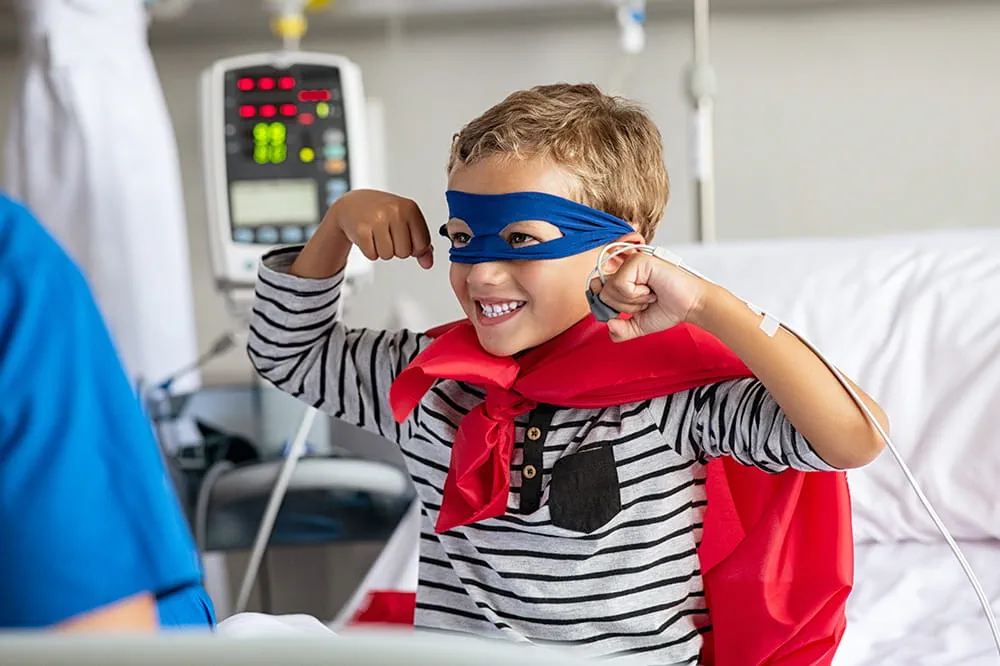
Ranked nationally in pediatric care.
Arkansas Children's provides right-sized care for your child. U.S. News & World Report has ranked Arkansas Children's in seven specialties for 2025-2026.

It's easier than ever to sign up for MyChart.
Sign up online to quickly and easily manage your child's medical information and connect with us whenever you need.

We're focused on improving child health through exceptional patient care, groundbreaking research, continuing education, and outreach and prevention.

When it comes to your child, every emergency is a big deal.
Our ERs are staffed 24/7 with doctors, nurses and staff who know kids best – all trained to deliver right-sized care for your child in a safe environment.

Arkansas Children's provides right-sized care for your child. U.S. News & World Report has ranked Arkansas Children's in seven specialties for 2025-2026.

Looking for resources for your family?
Find health tips, patient stories, and news you can use to champion children.

Support from the comfort of your home.
Our flu resources and education information help parents and families provide effective care at home.

Children are at the center of everything we do.
We are dedicated to caring for children, allowing us to uniquely shape the landscape of pediatric care in Arkansas.

Transforming discovery to care.
Our researchers are driven by their limitless curiosity to discover new and better ways to make these children better today and healthier tomorrow.

We're focused on improving child health through exceptional patient care, groundbreaking research, continuing education, and outreach and prevention.
Then we're looking for you! Work at a place where you can change lives...including your own.

When you give to Arkansas Children's, you help deliver on our promise of a better today and a healthier tomorrow for the children of Arkansas and beyond

Become a volunteer at Arkansas Children's.
The gift of time is one of the most precious gifts you can give. You can make a difference in the life of a sick child.

Join our Grassroots Organization
Support and participate in this advocacy effort on behalf of Arkansas’ youth and our organization.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.
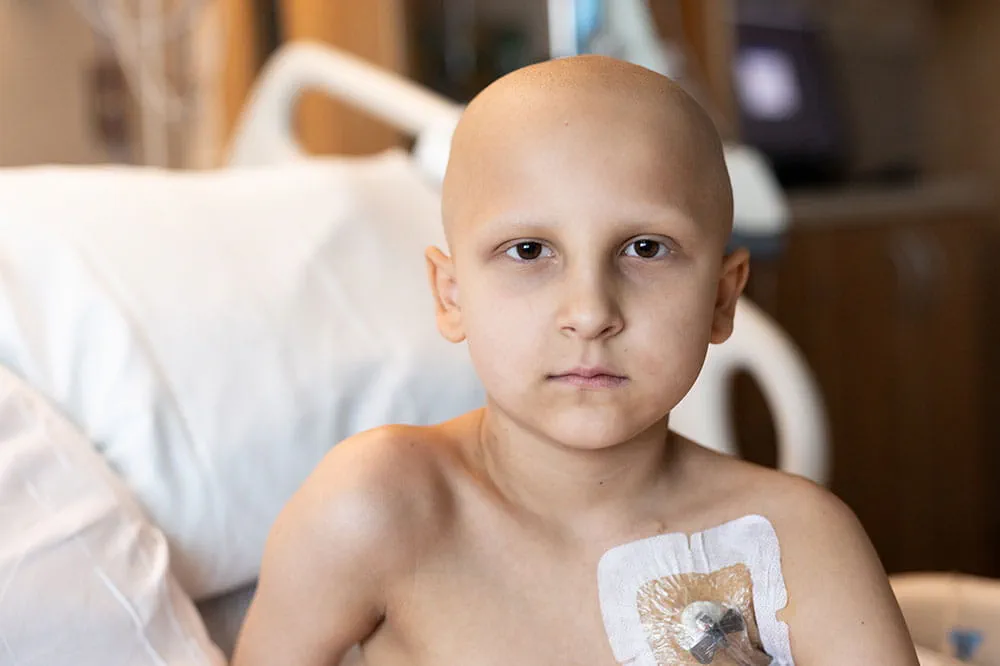
When you give to Arkansas Children’s, you help deliver on our promise of a better today and a healthier tomorrow for the children of Arkansas and beyond.

Your volunteer efforts are very important to Arkansas Children's. Consider additional ways to help our patients and families.

Join one of our volunteer groups.
There are many ways to get involved to champion children statewide.

Make a positive impact on children through philanthropy.
The generosity of our supporters allows Arkansas Children's to deliver on our promise of making children better today and a healthier tomorrow.

Read and watch heart-warming, inspirational stories from the patients of Arkansas Children’s.

Hello.

Arkansas Children's Hospital
General Information 501-364-1100
Arkansas Children's Northwest
General Information 479-725-6800

Arkansas Children's Hospital Becomes Nation's First Therapeutic Site in Launch of Groundbreaking Clinical Trial for Pediatric Acute Leukemia
June 29, 2022
LITTLE ROCK, AR. (June 29, 2022) – Arkansas Children's Hospital (ACH) will be the first site in the nation to enroll patients in a therapeutic clinical trial designed to fundamentally change how children with acute leukemias are treated. The Leukemia & Lymphoma Society launches the worldwide Pediatric Acute Leukemia (PedAL) Master Clinical Trial this summer to expedite the development of new targeted treatments for hard-to-treat childhood leukemias and replace one-size-fits-all chemotherapy with therapies tailored to each child's unique disease.
Before enrolling in therapeutic trials, parents can enroll a child in the PedAL screening trial to identify the unique tumor biology of each child's cancer and help them identify the most promising treatment for their specific type of leukemia. Arkansas kids fighting leukemia will have access to PedAL's therapeutic trial at Arkansas Children's Hospital in Little Rock, and patients will be able to enroll in the screening trial and have follow-ups at Arkansas Children's Northwest (ACNW) in Springdale. Through the support of the Arkansas Children's Research Institute (ACRI), ACH can rapidly initiate important clinical trials to offer cutting-edge therapies and ensure unprecedented health for all children in the region.
"PedAL will be a paradigm-changing clinical study and will provide so much hope for families of children with relapsed and hard-to-treat leukemias," said Dr. Jason Farrar, a PedAL investigator who directs the leukemia and lymphoma program at Arkansas Children's. He is also an associate professor in the Department of Pediatrics in the College of Medicine at the University of Arkansas for Medical Sciences (UAMS) and a member of the UAMS Winthrop P. Rockefeller Cancer Institute. "Kids are not little adults. They need safe and effective cancer treatments developed specifically for them, which is what PedAL is doing."
The trial also highlights advancements in cancer care for Arkansas kids, as the Cancer and Blood Disorders service at Arkansas Children's Hospital — recently ranked among U.S. News & World Report's best pediatric cancer programs — delivers more innovative treatments historically only available to families out of state.
"Keeping these children near their homes and families during cancer care is crucial," Farrar said. "By offering this trial, we are giving kids with leukemias more opportunities to not only survive, but truly thrive."
Because blood cancers are more common in adults, there is a larger incentive for new treatments to be developed in that population, and progress for pediatric acute leukemia has fallen behind. For children, including those with aggressive forms of cancer, a delay in research and therapies can threaten their survival. Only 69% of kids with acute myeloid leukemia (AML) will survive more than five years. Even when treatments are effective, more than 70% of childhood cancer survivors have a chronic health condition and 42% have a severe, disabling or life-threatening condition 30 years after diagnosis.
Through partnerships with the National Cancer Institute (NCI), part of the National Institutes of Health (NIH); Children's Oncology Group (COG); and the European Pediatric Acute Leukemia (EuPAL) Foundation, PedAL trials will be available to children and families worldwide – bringing the dream of safer, more effective pediatric leukemia treatments closer to home for more people.
Multiple screening trial sites are open in Canada and the United States. More therapeutic sites will open throughout 2022 and into the next year. In partnership with the University of Chicago, the Data Commons will consolidate pediatric clinical trial data from multiple institutions into a single, unified data set to ensure consistency in data collection, analysis and reporting. In addition, GEARBOx (Genomic Eligibility Algorithm at Relapse for Better Outcomes) is a unique search tool that will help healthcare professionals match patients who have relapsed or refractory disease to appropriate clinical trials and lifesaving treatments.
By enrolling their child in a screening trial, parents help advance research for all children because their child's tumor biology results will be assessed alongside children around the globe to help researchers find patterns. Parents who want to learn more can visit https://bloodcancerunited.org/ or call 1-800-955-4572 to reach the Information Resource Center.
About Arkansas Children's
Arkansas Children's is the only healthcare system in the state solely dedicated to caring for Arkansas' more than 700,000 children. The private, non-profit organization includes two pediatric hospitals, a pediatric research institute and USDA nutrition center, a philanthropic foundation, a nursery alliance, statewide clinics, and many education and outreach programs — all focused on fulfilling a promise to define and deliver unprecedented child health. Arkansas Children's Hospital (ACH) is a 336-bed, Magnet-recognized facility in Little Rock operating the state's only Level I pediatric trauma center; the state's only burn center; the state's only Level IV neonatal intensive care unit; the state's only pediatric intensive care unit; the state's only pediatric surgery program with Level 1 verification from the American College of Surgeons (ACS); the state's only magnetoencephalography (MEG) system for neurosurgical planning and cutting-edge research; and the state's only nationally recognized pediatric transport program. Arkansas Children's Hospital is nationally ranked by U.S. News & World Report in seven pediatric specialties (2022—2023): Cancer, Cardiology & Heart Surgery, Diabetes & Endocrinology, Nephrology, Neurology & Neurosurgery, Pulmonology and Urology. Arkansas Children's Northwest (ACNW), the first and only pediatric hospital in the Northwest Arkansas region, is a level IV pediatric trauma center. ACNW operates a 24-bed inpatient unit; a surgical unit with five operating rooms; outpatient clinics offering over 20 subspecialties; diagnostic services; imaging capabilities; occupational therapy services; and Northwest Arkansas' only pediatric emergency department, equipped with 30 exam rooms. Generous philanthropic and volunteer engagement has sustained Arkansas Children's since it began as an orphanage in 1912, and today ensures the system can deliver on its promise of unprecedented child health. To learn more, visit archildrens.org.
About UAMS
UAMS is the state's only health sciences university, with colleges of Medicine, Nursing, Pharmacy, Health Professions and Public Health; a graduate school; a hospital; a main campus in Little Rock; a Northwest Arkansas regional campus in Fayetteville; a statewide network of regional campuses; and seven institutes: the Winthrop P. Rockefeller Cancer Institute, Jackson T. Stephens Spine & Neurosciences Institute, Harvey & Bernice Jones Eye Institute, Psychiatric Research Institute, Donald W. Reynolds Institute on Aging, Translational Research Institute and Institute for Digital Health & Innovation. UAMS includes UAMS Health, a statewide health system that encompasses all of UAMS' clinical enterprise. UAMS is the only adult Level 1 trauma center in the state. U.S. News & World Report recognized UAMS Medical Center as a Best Hospital for 2021-22; ranked its ear, nose and throat program among the top 50 nationwide for the third year; and named five areas as high performing — colon cancer surgery, diabetes, hip replacement, knee replacement and stroke. UAMS has 3,047 students, 873 medical residents and fellows, and six dental residents. It is the state's largest public employer with more than 11,000 employees, including 1,200 physicians who provide care to patients at UAMS, its regional campuses, Arkansas Children's, the VA Medical Center and Baptist Health. Visit www.uams.edu or www.uamshealth.com. Find us on Facebook, Twitter, YouTube or Instagram.
About The Leukemia & Lymphoma Society
The Leukemia & Lymphoma Society® (LLS) is a global leader in the fight against blood cancer. The LLS mission: Cure leukemia, lymphoma, Hodgkin's disease and myeloma, and improve the quality of life of patients and their families. LLS funds lifesaving blood cancer research around the world, provides free information and support services, and is the voice for all blood cancer patients seeking access to quality, affordable, coordinated care. Founded in 1949 and headquartered in Rye Brook, NY, LLS has regions throughout the United States and Canada. To learn more, visit www.LLS.org. Patients should contact the Information Resource Center at (800) 955-4572, Monday through Friday, 9 a.m. to 9 p.m. ET. For additional information visit www.lls.org/llsnewsnetwork. Follow us on Facebook, Twitter and Instagram.
# # #

