Protect your family from respiratory illnesses. Schedule your immunization here >
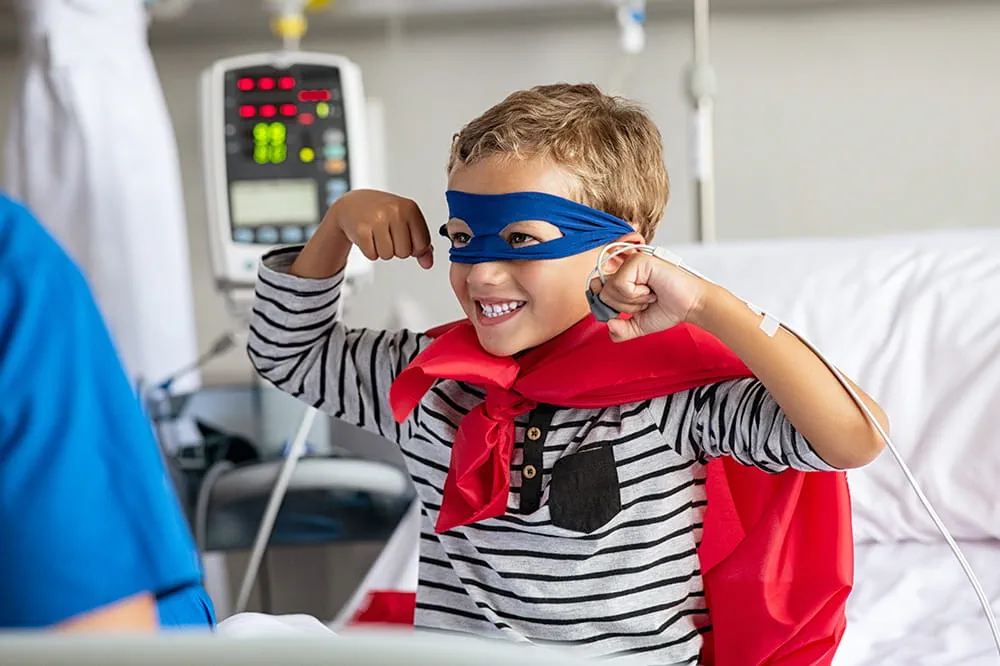
Ranked nationally in pediatric care.
Arkansas Children's provides right-sized care for your child. U.S. News & World Report has ranked Arkansas Children's in seven specialties for 2025-2026.

It's easier than ever to sign up for MyChart.
Sign up online to quickly and easily manage your child's medical information and connect with us whenever you need.
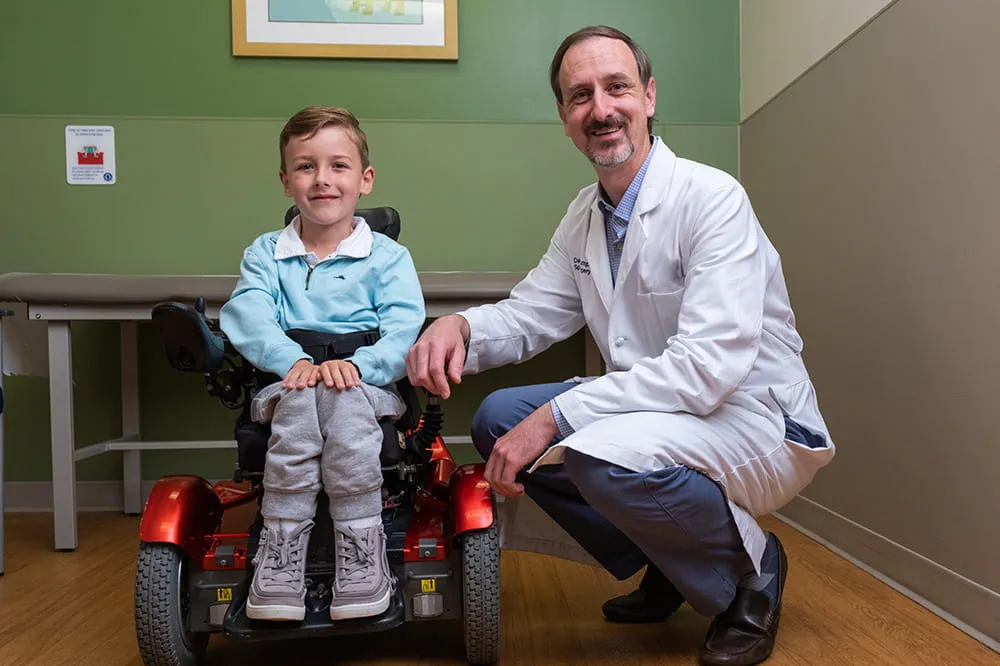
We're focused on improving child health through exceptional patient care, groundbreaking research, continuing education, and outreach and prevention.

When it comes to your child, every emergency is a big deal.
Our ERs are staffed 24/7 with doctors, nurses and staff who know kids best – all trained to deliver right-sized care for your child in a safe environment.

Arkansas Children's provides right-sized care for your child. U.S. News & World Report has ranked Arkansas Children's in seven specialties for 2025-2026.

Looking for resources for your family?
Find health tips, patient stories, and news you can use to champion children.

Support from the comfort of your home.
Our flu resources and education information help parents and families provide effective care at home.

Children are at the center of everything we do.
We are dedicated to caring for children, allowing us to uniquely shape the landscape of pediatric care in Arkansas.

Transforming discovery to care.
Our researchers are driven by their limitless curiosity to discover new and better ways to make these children better today and healthier tomorrow.

We're focused on improving child health through exceptional patient care, groundbreaking research, continuing education, and outreach and prevention.
Then we're looking for you! Work at a place where you can change lives...including your own.

When you give to Arkansas Children's, you help deliver on our promise of a better today and a healthier tomorrow for the children of Arkansas and beyond

Become a volunteer at Arkansas Children's.
The gift of time is one of the most precious gifts you can give. You can make a difference in the life of a sick child.

Join our Grassroots Organization
Support and participate in this advocacy effort on behalf of Arkansas’ youth and our organization.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.
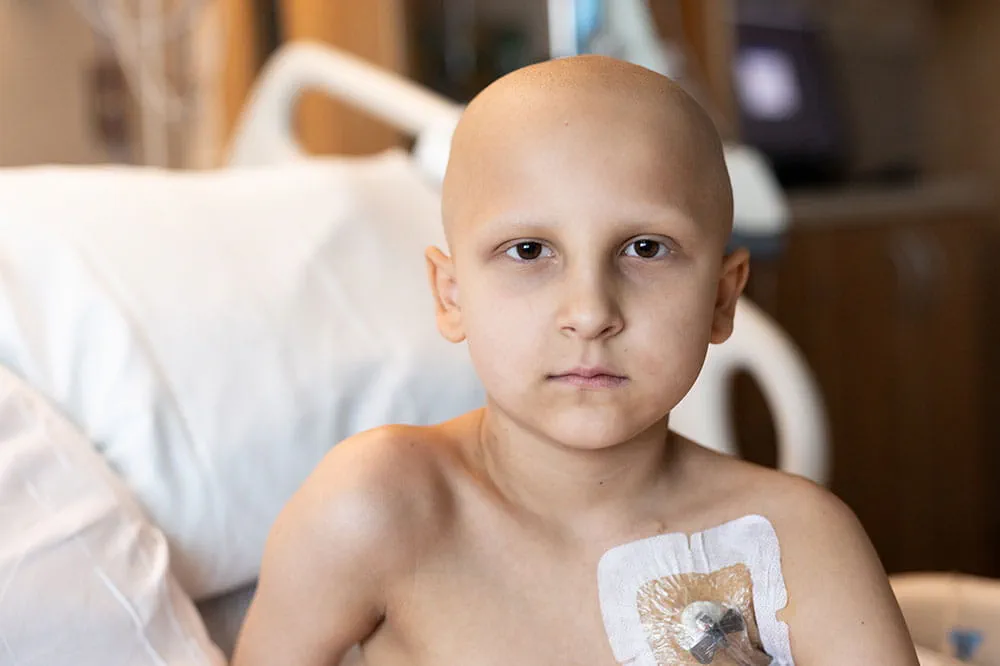
When you give to Arkansas Children’s, you help deliver on our promise of a better today and a healthier tomorrow for the children of Arkansas and beyond.

Your volunteer efforts are very important to Arkansas Children's. Consider additional ways to help our patients and families.

Join one of our volunteer groups.
There are many ways to get involved to champion children statewide.

Make a positive impact on children through philanthropy.
The generosity of our supporters allows Arkansas Children's to deliver on our promise of making children better today and a healthier tomorrow.

Read and watch heart-warming, inspirational stories from the patients of Arkansas Children’s.

Hello.

Arkansas Children's Hospital
General Information 501-364-1100
Arkansas Children's Northwest
General Information 479-725-6800

Oral Immunotherapy Trial Induces Remission of Peanut Allergy in Some Young Children
Arkansas Children’s Research Institute Among Five Sites in NIH Trial Published in The Lancet
January 21, 2022
LITTLE ROCK, AR. (Jan. 21, 2022) – A clinical trial funded by the National Institutes of Health has found that giving peanut oral immunotherapy to highly peanut-allergic children ages 1 to 3 years safely desensitized most of them to peanuts and induced remission of peanut allergy in one-fifth. The results of the trial, co-led by Arkansas Children’s Research Institute (ACRI) and University of Arkansas for Medical Sciences (UAMS) researcher Stacie Jones, M.D., are published today in the journal The Lancet.
ACRI is among five U.S. academic medical centers that participated in the trial. Nearly 150 children ages 1 to 3 years participated in the IMPACT trial at the five sites. Only children who had an allergic reaction after eating half a gram of peanut protein (about 1.5 peanuts) or less were eligible to join the study.
The immunotherapy studied consisted of a daily oral dose of peanut protein flour for 2.5 years. Remission was defined as being able to eat 5 grams of peanut protein, equivalent to approximately 16 peanuts, without having an allergic reaction six months after completing immunotherapy. The youngest children and those who started the trial with lower levels of peanut-specific antibodies were most likely to achieve remission.
Jones, director of the Arkansas Children’s Food Allergy Program and a UAMS professor of pediatrics practicing at Arkansas Children’s Hospital (ACH), served as protocol co-chair for the trial.
“The findings from the IMPACT Trial are important and showed that intervening early in the life of a peanut-allergic child may be more effective than intervening later in life. Specifically, a subset of study participants were able to safely consume 16 peanuts without symptoms at the end of the study. We are beyond grateful to the children and families who took a risk to be a part of the studies that led to this discovery,” Jones said. “Their contribution is huge and helps us lay the groundwork for a new therapy that may eventually help young children with peanut allergy live with less risk every day.”
Peanut allergy affects 2 percent of children in Western countries and most remain allergic across their lifetime, according to the medical literature. Current standard of care for young children with peanut allergy is dietary avoidance, but the risk of peanut-induced anaphylaxis from accidental exposures remains significant, highlighting the need for safe and effective therapies.
“In addition to the desensitization we saw in a majority of peanut-allergic children, we also saw a promising number of children whose peanut tolerability did not reach our threshold for remission but still increased significantly after treatment,” Jones said. “We are tremendously excited that some of this work happened at Arkansas Children’s Research Institute, underscoring our commitment to discoveries that have life-changing potential for families.”
The NIH-funded Immune Tolerance Network conducted the trial under the leadership of A. Wesley Burks, M.D., and Dr. Jones. Dr. Burks is chief executive officer of UNC Health Care, dean of the UNC School of Medicine, and vice chancellor for medical affairs at the University of North Carolina at Chapel Hill. The other participating institutions included Stanford University, Johns Hopkins Hospital and Icahn School of Medicine at Mount Sinai.
ABOUT ARKANSAS CHILDREN’S
Arkansas Children's, Inc. is the only healthcare system in the state solely dedicated to caring for Arkansas' more than 700,000 children. The private, non-profit organization includes two pediatric hospitals, a pediatric research institute and USDA nutrition center, a philanthropic foundation, a nursery alliance, statewide clinics, and many education and outreach programs — all focused on fulfilling a promise to define and deliver unprecedented child health. Arkansas Children’s Hospital (ACH) is a 336-bed, Magnet-recognized facility in Little Rock operating the state’s only Level I pediatric trauma center; the state's only burn center; the state's only Level IV neonatal intensive care unit; the state's only pediatric intensive care unit; the state’s only pediatric surgery program with Level 1 verification from the American College of Surgeons (ACS); the state’s only magnetoencephalography (MEG) system for neurosurgical planning and cutting-edge research; and the state's only nationally recognized pediatric transport program. Additionally, ACH is nationally ranked by U.S. News & World Report in four pediatric subspecialties (2021—2022): Cardiology & Heart Surgery, Nephrology, Pulmonology and Urology. Arkansas Children’s Northwest (ACNW), the first and only pediatric hospital in the Northwest Arkansas region, is a level IV pediatric trauma center. ACNW operates a 24-bed inpatient unit; a surgical unit with five operating rooms; outpatient clinics offering over 20 subspecialties; diagnostic services; imaging capabilities; occupational therapy services; and Northwest Arkansas' only pediatric emergency department, equipped with 30 exam rooms. Generous philanthropic and volunteer engagement has sustained Arkansas Children's since it began as an orphanage in 1912, and today ensures the system can deliver on its promise of unprecedented child health. To learn more, visit archildrens.org
ABOUT UAMS
UAMS is the state's only health sciences university, with colleges of Medicine, Nursing, Pharmacy, Health Professions and Public Health; a graduate school; a hospital; a main campus in Little Rock; a Northwest Arkansas regional campus in Fayetteville; a statewide network of regional campuses; and seven institutes: the Winthrop P. Rockefeller Cancer Institute, Jackson T. Stephens Spine & Neurosciences Institute, Harvey & Bernice Jones Eye Institute, Psychiatric Research Institute, Donald W. Reynolds Institute on Aging, Translational Research Institute and Institute for Digital Health & Innovation. UAMS includes UAMS Health, a statewide health system that encompasses all of UAMS' clinical enterprise. UAMS is the only adult Level 1 trauma center in the state. U.S. News & World Report recognized UAMS Medical Center as a Best Hospital for 2021-22; ranked its ear, nose and throat program among the top 50 nationwide for the third year; and named five areas as high performing — colon cancer surgery, diabetes, hip replacement, knee replacement and stroke. Forbes magazine ranked UAMS as seventh in the nation on its Best Employers for Diversity list. UAMS also ranked in the top 30% nationwide on Forbes’ Best Employers for Women list and was the only Arkansas employer included. UAMS has 3,047 students, 873 medical residents and fellows, and six dental residents. It is the state's largest public employer with more than 10,000 employees, including 1,200 physicians who provide care to patients at UAMS, its regional campuses, Arkansas Children's, the VA Medical Center and Baptist Health. Visit www.uams.edu or www.uamshealth.com. Find us on Facebook, Twitter, YouTube or Instagram.
# # #

