Protect your family from respiratory illnesses. Schedule your immunization here >
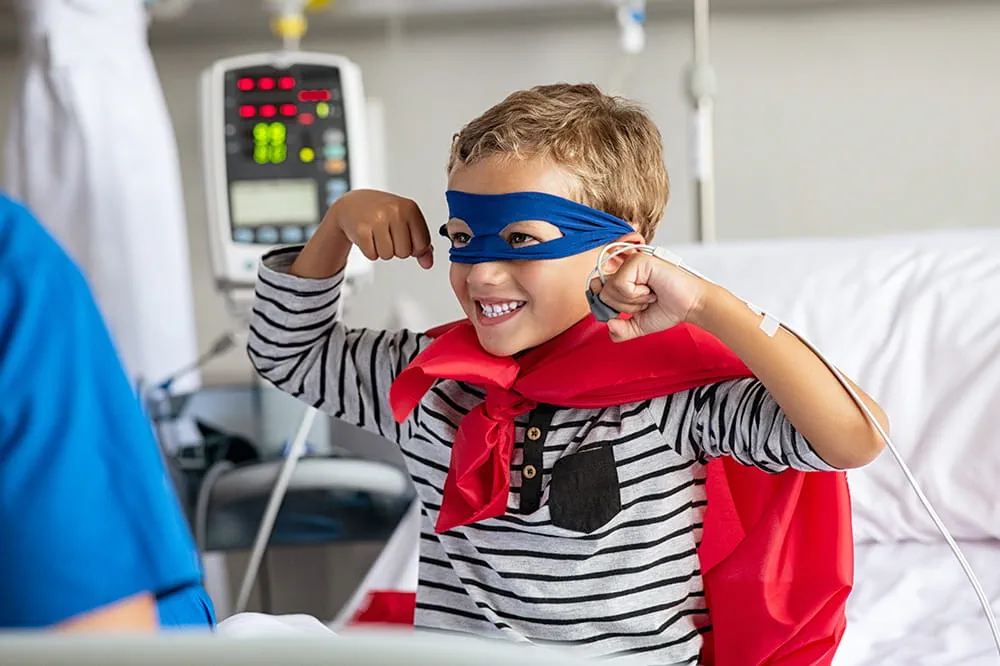
Ranked nationally in pediatric care.
Arkansas Children's provides right-sized care for your child. U.S. News & World Report has ranked Arkansas Children's in seven specialties for 2025-2026.

It's easier than ever to sign up for MyChart.
Sign up online to quickly and easily manage your child's medical information and connect with us whenever you need.
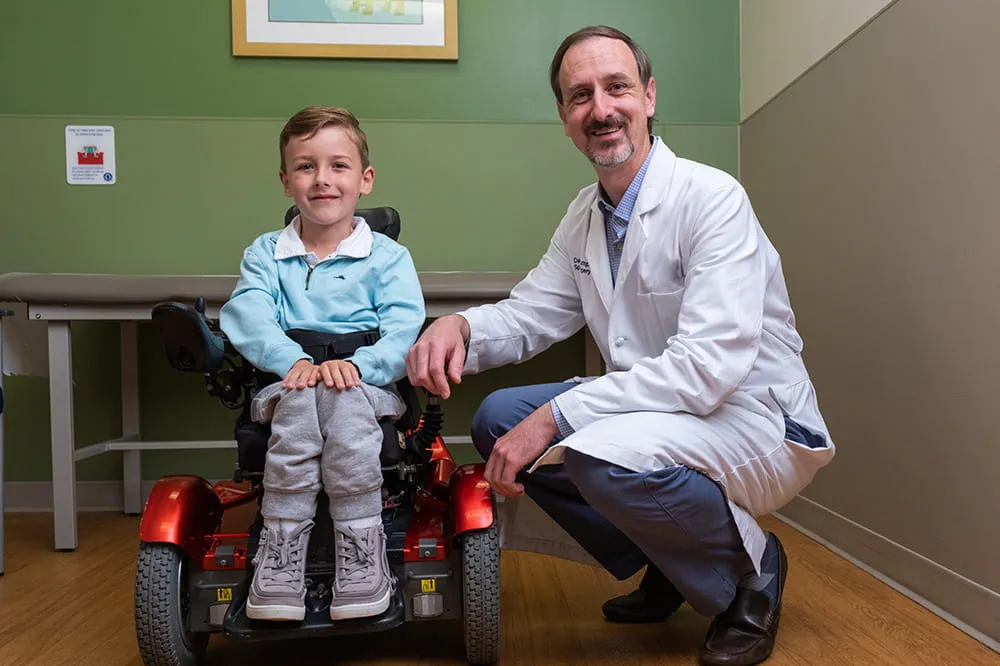
We're focused on improving child health through exceptional patient care, groundbreaking research, continuing education, and outreach and prevention.

When it comes to your child, every emergency is a big deal.
Our ERs are staffed 24/7 with doctors, nurses and staff who know kids best – all trained to deliver right-sized care for your child in a safe environment.

Arkansas Children's provides right-sized care for your child. U.S. News & World Report has ranked Arkansas Children's in seven specialties for 2025-2026.

Looking for resources for your family?
Find health tips, patient stories, and news you can use to champion children.

Support from the comfort of your home.
Our flu resources and education information help parents and families provide effective care at home.

Children are at the center of everything we do.
We are dedicated to caring for children, allowing us to uniquely shape the landscape of pediatric care in Arkansas.

Transforming discovery to care.
Our researchers are driven by their limitless curiosity to discover new and better ways to make these children better today and healthier tomorrow.

We're focused on improving child health through exceptional patient care, groundbreaking research, continuing education, and outreach and prevention.
Then we're looking for you! Work at a place where you can change lives...including your own.

When you give to Arkansas Children's, you help deliver on our promise of a better today and a healthier tomorrow for the children of Arkansas and beyond

Become a volunteer at Arkansas Children's.
The gift of time is one of the most precious gifts you can give. You can make a difference in the life of a sick child.

Join our Grassroots Organization
Support and participate in this advocacy effort on behalf of Arkansas’ youth and our organization.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.
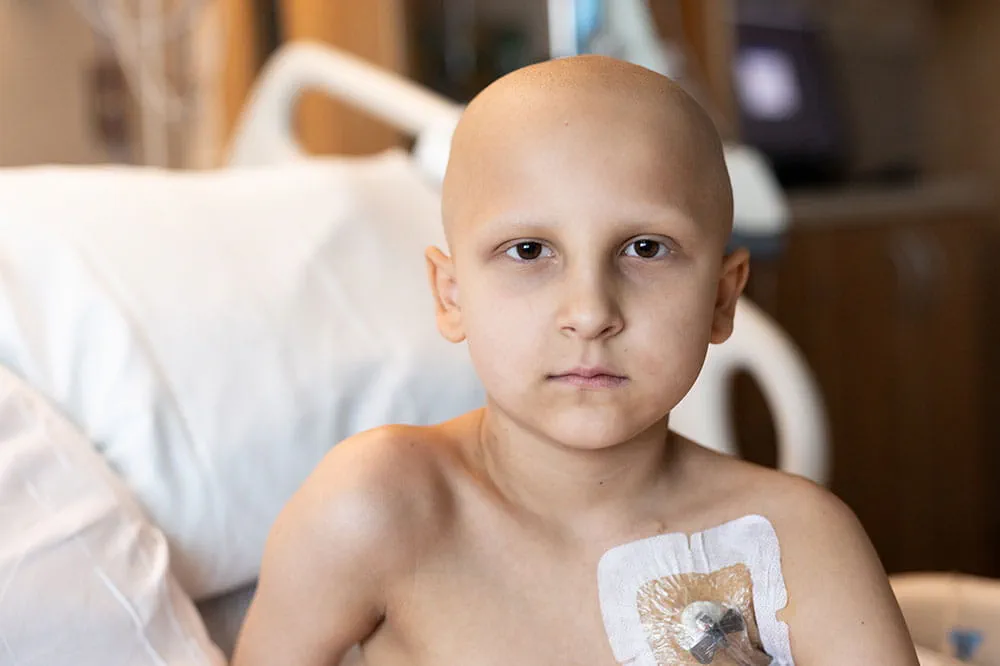
When you give to Arkansas Children’s, you help deliver on our promise of a better today and a healthier tomorrow for the children of Arkansas and beyond.

Your volunteer efforts are very important to Arkansas Children's. Consider additional ways to help our patients and families.

Join one of our volunteer groups.
There are many ways to get involved to champion children statewide.

Make a positive impact on children through philanthropy.
The generosity of our supporters allows Arkansas Children's to deliver on our promise of making children better today and a healthier tomorrow.

Read and watch heart-warming, inspirational stories from the patients of Arkansas Children’s.

Hello.

Arkansas Children's Hospital
General Information 501-364-1100
Arkansas Children's Northwest
General Information 479-725-6800

A Phase 2 Study to Assess the Safety and Efficacy of Cobimetinib in Refractory Langerhans Cell Histiocytosis, LCH-Associated Neurodegenerative Disease, and Other Histiocytic Disorders
Summary and Objectives
Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplasm, which means it is a disease where certain bone marrow-related immune cells grow abnormally and cause inflammation in the body, which can damage tissues in areas like bone, skin, and organs. In the past, treatment options have been limited, and are often associated with high toxicity. With advances in understanding of LCH biology, new targeted or cellular therapies may ultimately be more effective and/or safer than high-dose chemotherapy for patients who fail to respond to initial treatments.
Cobimetinib is a MEK inhibitor, which is a type of targeted therapy that blocks the activity of certain enzymes responsible for cell survival, division, and growth. Supported by the success of targeted therapies in studies of similar context, this study seeks to evaluate the safety and efficacy of Cobimetinib as a less toxic treatment option for certain histiocytic disorders.
Eligibility criteria
In addition to a few criteria that could prevent participation, there are criteria participants must meet including but not limited to the following:
- For groups 1-3, participants must be at least 6 months old; group 4 participants must be at least 21 years old.
- Must be able to take an oral suspension or tablet which may be taken by mouth or other enteral route such as nasogastric or gastric tube.
- Must have a biopsy-confirmed qualifying diagnosis
- Must have failed at least initial line of therapy for patients with LCH
- Participants with LCH-associated neurodegenerative disease must have confirmed radiologic or clinical progression within the past 3 months
- Must have adequate organ function and performance levels as determined by the study protocol
- Female patients of childbearing potential require a negative urine or serum pregnancy test and must agree to follow the contraceptive requirements using two forms of effective contraceptive methods for the duration of the study treatment.
- Male patients with sexual partners who are pregnant or who could become pregnant (i.e., women of child-bearing potential) must agree to use two forms of effective methods of contraception (one of which must be a barrier method) during the treatment period and for at least 3 months after the last dose of the study drug.
- Must not continue taking certain protocol-identified medications/therapies
- Must not have received Cobimetinib previously
- Various protocol-identified comorbidities (active or history of) could prevent participation in this study
Clinicaltrials.gov Study Details >
Contact Information: For more information, contact Ashley Bryan at 501-364-3122 or Suzy Hall at 501-364-4181.
Principal Investigator


