Protect your family from respiratory illnesses. Schedule your immunization here >
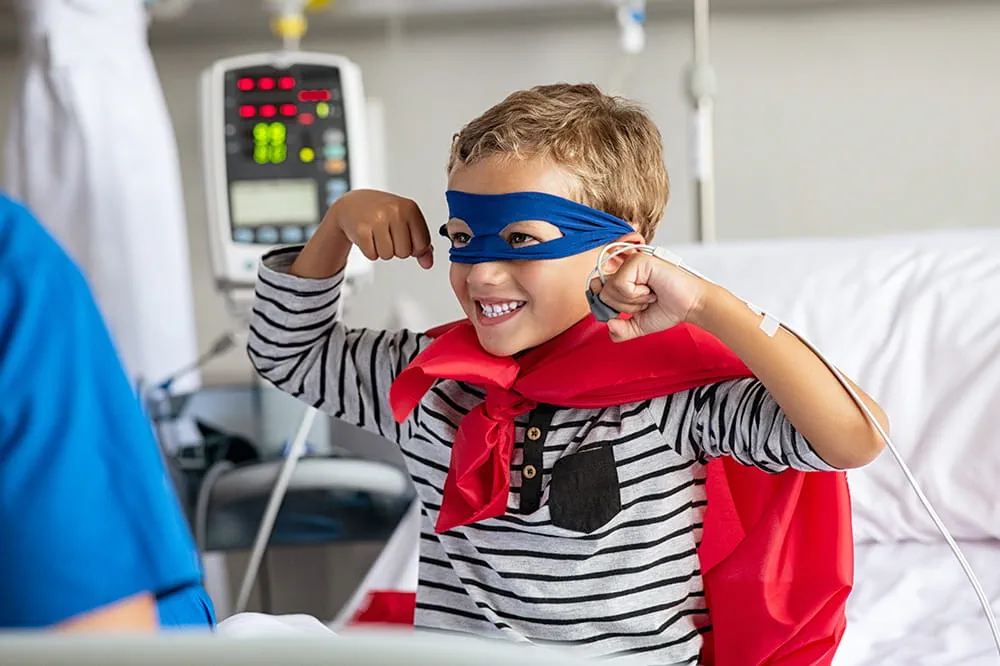
Ranked nationally in pediatric care.
Arkansas Children's provides right-sized care for your child. U.S. News & World Report has ranked Arkansas Children's in seven specialties for 2025-2026.

It's easier than ever to sign up for MyChart.
Sign up online to quickly and easily manage your child's medical information and connect with us whenever you need.

We're focused on improving child health through exceptional patient care, groundbreaking research, continuing education, and outreach and prevention.

When it comes to your child, every emergency is a big deal.
Our ERs are staffed 24/7 with doctors, nurses and staff who know kids best – all trained to deliver right-sized care for your child in a safe environment.

Arkansas Children's provides right-sized care for your child. U.S. News & World Report has ranked Arkansas Children's in seven specialties for 2025-2026.

Looking for resources for your family?
Find health tips, patient stories, and news you can use to champion children.

Support from the comfort of your home.
Our flu resources and education information help parents and families provide effective care at home.

Children are at the center of everything we do.
We are dedicated to caring for children, allowing us to uniquely shape the landscape of pediatric care in Arkansas.

Transforming discovery to care.
Our researchers are driven by their limitless curiosity to discover new and better ways to make these children better today and healthier tomorrow.

We're focused on improving child health through exceptional patient care, groundbreaking research, continuing education, and outreach and prevention.
Then we're looking for you! Work at a place where you can change lives...including your own.

When you give to Arkansas Children's, you help deliver on our promise of a better today and a healthier tomorrow for the children of Arkansas and beyond

Become a volunteer at Arkansas Children's.
The gift of time is one of the most precious gifts you can give. You can make a difference in the life of a sick child.

Join our Grassroots Organization
Support and participate in this advocacy effort on behalf of Arkansas’ youth and our organization.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.

Learn How We Transform Discovery to Care
Scientific discoveries lead us to new and better ways to care for children.
When you give to Arkansas Children’s, you help deliver on our promise of a better today and a healthier tomorrow for the children of Arkansas and beyond.

Your volunteer efforts are very important to Arkansas Children's. Consider additional ways to help our patients and families.

Join one of our volunteer groups.
There are many ways to get involved to champion children statewide.

Make a positive impact on children through philanthropy.
The generosity of our supporters allows Arkansas Children's to deliver on our promise of making children better today and a healthier tomorrow.

Read and watch heart-warming, inspirational stories from the patients of Arkansas Children’s.

Hello.

Arkansas Children's Hospital
General Information 501-364-1100
Arkansas Children's Northwest
General Information 479-725-6800

Arkansas ECHO ISPCTN Site
 The vast majority of research conducted in children in the United States (US) today does not include children living in the 24 IDeA eligible states/territories identified by the National Institutes of Health such as Arkansas. Consequently, findings may not be generalizable to a substantial proportion of children who carry the highest disease burdens such as obesity, diabetes, pre-maturity, lung and neurodevelopmental disorders. Future therapeutic and non- therapeutic trials must be available to these children if their health status is to be improved. The Arkansas Children's Research Institute is prepared to immediately address the overarching program goals of the IDeA States Pediatric Clinical Trial Network (ISPCTN) and thereby, work to close the "clinical trial gap" for children living in IDeA states. The Arkansas ECHO ISPCTN Site (AREIS) was formed to enable the participation of diverse groups of infants, children and adolescents in expertly designed, innovative clinical trials of the highest quality and scientific value. We will leverage our unique patient base, intellectual capital and established inter-institutional resources to enhance pediatric clinical trials funded by the NIH and other supported research. Our established clinical trial enterprise and cadre of senior pediatric clinical investigators will enable us to explore the impact and intersection of ontogeny, environmental exposure and disease in four high priority areas: upper and lower airway disease; obesity; neurodevelopment and pre-, peri- and postnatal development. AREIS will improve the health of children of Arkansas and beyond by systematically addressing the unique health challenges of IDeA state populations.
The vast majority of research conducted in children in the United States (US) today does not include children living in the 24 IDeA eligible states/territories identified by the National Institutes of Health such as Arkansas. Consequently, findings may not be generalizable to a substantial proportion of children who carry the highest disease burdens such as obesity, diabetes, pre-maturity, lung and neurodevelopmental disorders. Future therapeutic and non- therapeutic trials must be available to these children if their health status is to be improved. The Arkansas Children's Research Institute is prepared to immediately address the overarching program goals of the IDeA States Pediatric Clinical Trial Network (ISPCTN) and thereby, work to close the "clinical trial gap" for children living in IDeA states. The Arkansas ECHO ISPCTN Site (AREIS) was formed to enable the participation of diverse groups of infants, children and adolescents in expertly designed, innovative clinical trials of the highest quality and scientific value. We will leverage our unique patient base, intellectual capital and established inter-institutional resources to enhance pediatric clinical trials funded by the NIH and other supported research. Our established clinical trial enterprise and cadre of senior pediatric clinical investigators will enable us to explore the impact and intersection of ontogeny, environmental exposure and disease in four high priority areas: upper and lower airway disease; obesity; neurodevelopment and pre-, peri- and postnatal development. AREIS will improve the health of children of Arkansas and beyond by systematically addressing the unique health challenges of IDeA state populations.
AREIS is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number UG1OD024945.
Directors


